Thimerosal, a mercury-based preservative used in some flu shots, has returned to the center of controversy after a dramatic policy shift by a newly reshaped CDC vaccine advisory panel. On June 26, 2025, the panel voted to restrict thimerosal-containing influenza vaccines, raising questions not only about science and public health but also about legal authority, transparency, and global implications.
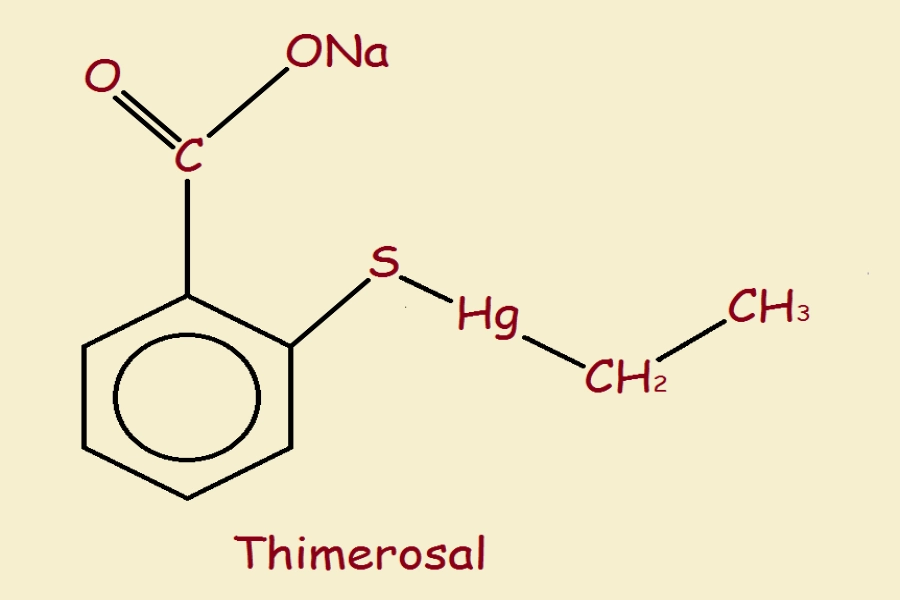
What Is Thimerosal?
Thimerosal is a preservative containing ethylmercury. It has been used in vaccines since the 1930s to prevent contamination in multi-dose vials. Its rapid breakdown in the body and short half-life distinguish it from methylmercury, the neurotoxin found in fish.
Scientific Consensus: Thimerosal Is Safe
Extensive research by organizations such as the CDC, WHO, and NIH confirms thimerosal’s safety. Multiple studies across different populations have found no evidence linking thimerosal to autism or other neurological conditions. Ethylmercury clears the body much faster than methylmercury and does not accumulate.
For perspective: a flu shot contains 25 micrograms of ethylmercury, less than a small can of tuna fish.
The CDC Vote: What Changed?
The Advisory Committee on Immunization Practices (ACIP) was restructured in June 2025 by U.S. Health Secretary Robert F. Kennedy Jr., a long-time vaccine skeptic. All 17 previous members were dismissed and replaced by new appointees, some lacking formal credentials or publications.
In a 5-1 vote (with one abstention), the panel recommended against thimerosal in all flu vaccines. A planned CDC presentation summarizing decades of safety data was withdrawn before the meeting.
Legal Analysis: Did the Panel Overstep?
1. Authority of the Secretary of Health
Under U.S. administrative law, the Health and Human Services Secretary has broad authority to appoint advisory panel members. However, dismissing the entire ACIP en masse raises questions about political interference in public health policymaking.
While the appointments are legally permissible, critics argue that such actions may violate norms of scientific independence, especially when driven by ideology rather than evidence.
2. Due Process and Transparency
The removal of CDC-authored presentations, along with the use of unverified data, may violate federal transparency and data quality guidelines, including:
-
Information Quality Act (IQA)
-
Federal Advisory Committee Act (FACA)
-
OMB Guidelines on Scientific Integrity
If litigation arises, courts may review whether the panel’s recommendations were “arbitrary or capricious” under the Administrative Procedure Act (APA).
3. Conflict of Interest & Accountability
As of the panel vote, new members had not filed standard conflict of interest disclosures. This failure undermines the committee’s credibility and could form the basis of legal challenges or Freedom of Information Act (FOIA) investigations.
Global Impact: Vaccine Access at Risk
Many countries rely on multi-dose vials containing thimerosal because they are cheaper, easier to store, and more efficient during mass immunization campaigns. If international agencies follow the U.S. lead, it could:
-
Increase vaccine production costs
-
Disrupt immunization programs
-
Reduce access in low-resource settings
As Dr. Cody Meissner noted, such a move may cause more harm than any hypothetical risk from thimerosal.
Misinformation Risk: Ideology Over Evidence?
Thimerosal has long been targeted by anti-vaccine activists, despite overwhelming scientific consensus about its safety. The ACIP vote, led by a panel aligned with anti-vaccine rhetoric, may inadvertently validate pseudoscientific claims and deepen public confusion.
Replacing peer-reviewed evidence with unvetted presentations—one of which cited a fabricated study—poses serious challenges to evidence-based policymaking.

Ethylmercury vs Methylmercury: Not the Same
| Property | Ethylmercury (Thimerosal) | Methylmercury (Seafood) |
|---|---|---|
| Toxicity | Low | High |
| Brain accumulation | No | Yes |
| Blood half-life | 7 days | 44–58 days |
| Found in vaccines? | Yes | No |
This difference is key to understanding why the fear around thimerosal is misplaced.
Policy Consequences
In the U.S.:
-
Clinicians may hesitate to use thimerosal-containing vaccines.
-
Manufacturers may preemptively stop producing them for U.S. markets.
-
Public trust may be further eroded.
Globally:
-
WHO and UNICEF may reevaluate procurement policies.
-
Supply chains may be disrupted.
-
Costs could rise for low-income countries.
Conclusion
The June 2025 CDC panel vote on thimerosal marks a sharp break from decades of science-based public health policy. While framed as a safety measure, the decision reflects broader ideological trends that may impact vaccine availability and trust—not just in the U.S., but worldwide.
It’s critical for journalists, scientists, and legal professionals to ensure accountability, transparency, and scientific integrity remain at the core of vaccine policymaking.
FAQ
Is thimerosal still used in vaccines?
Yes. It’s used in ~5% of flu vaccines in the U.S., mostly multi-dose vials.
Is thimerosal harmful?
No. It’s ethylmercury-based and clears quickly from the body.
Why was thimerosal removed from some vaccines?
As a precaution—not due to proven harm—it was phased out of pediatric vaccines in the early 2000s.
Does thimerosal cause autism?
No credible study has found any link between thimerosal and autism.
Could this policy affect global vaccine access?
Yes. Many countries rely on low-cost multi-dose vials that contain thimerosal.